Abstract
Introduction
Monoclonal antibody targeting CD38, daratumumab, has been shown to be a game changer and has generated focus among both patients and physicians. Major trials in patients with relapsed/refractory multiple myeloma (RRMM), CASTOR, POLLUX, APOLLO, and CANDOR, has proved benefits in both progression free survival (PFS) and response rate in favor of daratumumab incorporation into proteasome inhibitors (PI) or immunomodulatory agents (IMiDs) and dexamethasone doublet regimen. Daratumumab was also approved by the China Food and Drug Administration in the RR setting. Since then, it was applied in a number of combinations. However, the advantage of daratumumab combinations over conventional triplet regimens in real world was yet to clarified. Hereby, we retrospectively analyzed the efficacy of daratumumab-based regimens comparing with matched counterparts with conventional regimens as per physicians' choices using propensity score method in real-world patients with RRMM registered with the Hunan Guangdong Myeloma Working Group (HGMWG), a regional study group in South China.
Methods
Four hundreds eighty-six patients with RRMM were enrolled in this study, including 129 with daratumumab-based regimens and 357 with conventional regimens. Age, prior autologous stem cell transplantation, and drug resistance were balanced between groups using propensity score method. The primary endpoint was to evaluate the efficacy of daratumumab-based regimens, in terms of PFS, overall survival (OS), response rate according to the IMWG criteria. Cox proportional hazards regression models were used in univariate analyses to estimate the risk on survival outcomes.
Results
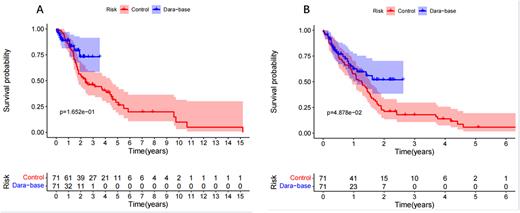
One hundred forty-two patients entered the analysis with a ratio of 1:1 between the daratumumab and the control arm (conventional regimens without daratumumab). The baseline characteristics were balanced expect that serum lactate dehydrogenase and extramedullary disease were higher in control arm. 29.58% patients were resistant to PIs, and 40.58% were double resistant to any kind of PIs and IMiDs. In control arm, 2 patients (2.8%) achieved sCR, 9 (12.7%) were CR, 3 (4.2%) were VGPR, and 12 (16.9%) were PR. The overall response rate was 36.6%. In daratumumab arm, 5 patients (7.0%) achieved sCR, 10 (14.1%) were CR, 13 (18.3%) were VGPR, and 12 (16.9%) were PR. The overall response rate was 57.1% (p=0.015). The median PFS time and the median OS time were 16.4 months and 37.8 months for the entire cohort. The median PFS time were 15.2 months in control arm versus unreached in daratumumab arm (p<0.05). The median OS time were 28.6 months in control arm versus unreached in daratumumab arm (p>0.05).
Conclusion In our real-world propensity score matched data, we confirmed that daratumumab incorporation of MM care could provide significant response and progression free survival benefits.
Figure legend: A. Progression free survival and B. overall survival of patients with RRMM receiving daratumumab-based regimens and conventional regimens (control arm).
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal